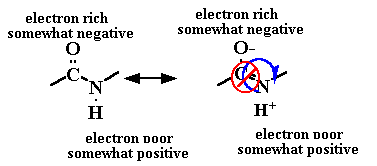
- Does not allow free rotation about the C-N bond
While the C-N bond of an amide is usually drawn as a single bond, it really is not in this case. There is "some double bond character" to it as shown to the left. One can think of the amide bond as somewhere in between these two pictures or as rapidly flipping from one to the other.
- Is almost always found in the "trans" configuration
Since there is no rotation then the relative "placement" of the H and the O atoms is fixed. They are almost always found facing away from each other in a "trans" configuration.
- The bond overall is polar
Just as in water the oxygen atom represents a more negatively charged side of the group while the H side of the group is more positively charged thus there is a polar moment to the entire functional group.

In the demonstration below you may "left click and drag" to rotate the molecule "SHIFT left click and drag (up or down)" to make smaller or bigger
Click on the white squares in succession to turn or/off identifying features. Some text describing the issues is in the lower right text box
  | |
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |
