 Triose Phosphate Isomerase Information
Triose Phosphate Isomerase Information Triose Phosphate Isomerase Information
Triose Phosphate Isomerase Information
Enzyme Name |
Triose Phosphate Isomerase | |
Reaction Catalyzed |
Isomerization between Dihydroxyacetone Phosphate and Glyceraldehyde-3-Phosphate
| |
Reaction Type |
Isomerization | |
Rationale |
The pieces from the aldol reaction are not identical, but are related to each other by a simple conversion. Another isomerization! On one of the halves the ketone is on C2 while on the other it is on C1. How can they be made to look identical? The glyceralhehyde does NOT NEED to do an ismomerization. ONLY dihydroxyacetone phosphate (ketone on C2) needs to be isomerized. Afterwards it is identical to the other half (glyceraldehyde-3-phosphate). FOR ALL REACTIONS FROM HERE ON, THERE ARE TWO MOLECULES GOING THROUGH EACH CONVERSION FOR EACH GLUCOSE THAT STARTED. | |
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
Cofactors/Cosubstrates |
None | |
DGo' |
+7.6 kJ/M in the direction of glycolysis |
Starting from standard state and allowing the reaction to come to equilibrium the dihydroxyacetone phosphate (DHAP) concentration would end up ~20 times higher than the concentration of glyceraldehyde-3-phosphate. The Standard Free Energy favors Fructose-1,6-bisphosphate production. |
Keq |
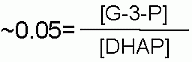 |
|
Comments |
This is the classic paradigm enzymefor isomerization reactions. It has been more well studied that almost any other enzyme. Even the protein fold (see below) is called the TIM β-barrel. TIM = Triose phosphate IsoMerase. Note the the barrel that is formed as β sheets structures wrap around the center of the protein.
| |
"In cell" Substrate Concentrations* |
||
S1 = |
dihydroxyacetone phosphate | 0.14 mM |
S2 = |
||
P1 = |
Glyceraldehyde-3-Phosphate | 0.019 mM |
P2 = |
||
DG for these conditions |
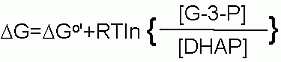 | |
+2.4 kJ/M | ||
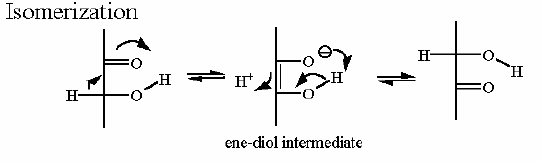
Mechanism for Chemistry |
 | |
Mechanism for Enzyme |
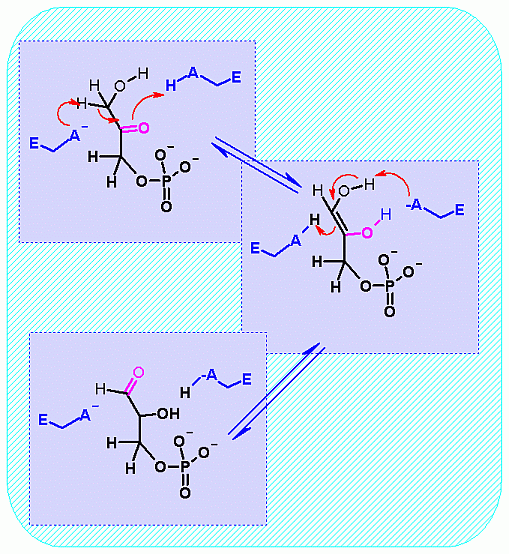
 Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Notice the "push - pull" of the base and acid to initiate the isomerzation to the "ene-diol" intermediate - and then they "reverse" their function to complete the reaction This reaction can obviously go both way and indeed the Standard Free Energy favors the DHAP substate. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. | |
Picture of Enzyme with substrate |
|
|
| ||
|
Phosphofructokinase PFK-1 CHIME representation | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.