 Enolase Information
Enolase Information Enolase Information
Enolase Information
|
Enzyme Name |
Enolase |
|
|
|
||
|
Reaction Catalyzed |
Elimination of water from 2-Phosphoglycerate
|
|
|
Reaction Type |
Elimination Reaction |
|
|
Rationale |
Elmination Reaction. The phosphate has already been placed on C2
of glycerate from the previous enzyme. The Enol functional group. The significance of the Enol group
because the terminal -H must be able to dissociate as H+ for the conversion.
which cannot dissociate therefore the molecule is trapped in
the less favorable enol form. |
|
|
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
|
Cofactors/Cosubstrates |
a metal ion usually Mg2+ is
required as a cofactor |
|
|
|
||
|
DGo' |
+23.9 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the Fructose-1,6-bisphosphate concentration would end up ~15,000 times higher than the product of the concetrations of DHAP and G-3-P. The Standard Free Energy favors Fructose-1,6-bisphosphate production. |
|
Keq |
 |
|
|
Comments |
Note: the Standard Free Energy grealy favors production of Fructose-1,6-bisphosphate. The mammalian forms of this enzyme go through a required covalently bound substrate for catalysis. This is true regardless direction of the reaction. Meaning, both directions go through through the same mechanism and utilize the same intermediate... as must be the case for all enzymes recations. |
|
|
"In cell" Substrate Concentrations* |
||
|
S1 = |
Fructose-1,6-bisPhosphate | 0.031 mM |
|
S2 = |
|
|
|
P1 = |
Dihydroyacetonephosphate (DHAP) | 0.14 mM |
|
P2 = |
Glyceraldehyde-3-Phosphate (G-3-P) |
0.019 mM |
|
DG for these conditions |
 |
|
|
-0.23 kJ/M |
||
|
Note this rather large turn around from DGo' to DG. This reaction does not have the same issues as that of a hydrolysis (water concentration at 50M/l)or that of an ATP dependent group transfer (ATP held fairly high and constant at 5mM). |
||
|
|
||
|
Mechanism for Chemistry |
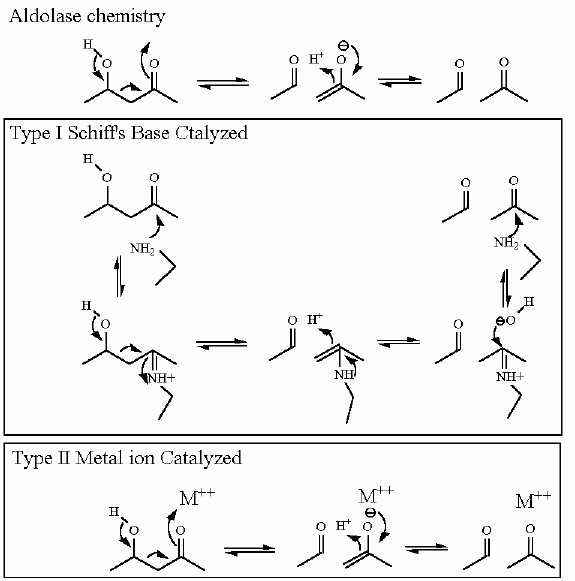 |
|
|
Mechanism for Enzyme |
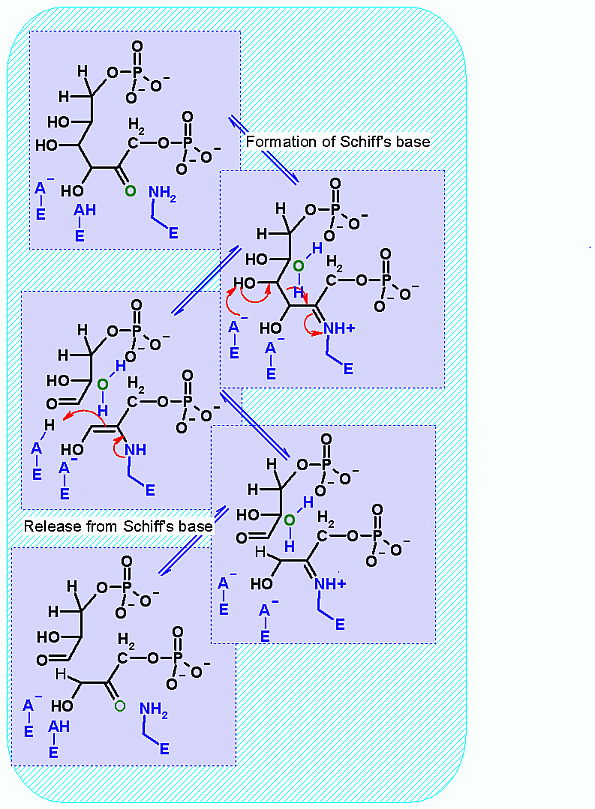
|
|
|
Picture of Enzyme with substrate |
|
|
|
|
|
|
|
Phosphofructokinase
PFK-1 CHIME representation |
|
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.