 Glycerate Kinase Information
Glycerate Kinase Information Glycerate Kinase Information
Glycerate Kinase Information
|
Enzyme Name |
Glycerate Kinase
|
|
|
|
||
|
Reaction Catalyzed |
Group Transfer from 1,3 bisphosphoglycerate
to ADP to make ATP
|
|
|
Reaction Type |
Group Transfer |
|
|
Rationale |
A group transfer of phosphate onto ADP to make ATP is made
thermodynamically favorable even starting from the standard state
because the phosphate is coming off of an organic acid. Please recall a
couple modules ago, a quiz question dealt with the Free Energies of a
phosphate hydrolysis from two different functional groups: 1. an
alcohol, 2. an organic acid; the Standard Free Energies differed
substantially at -17 kJ/M and -42 kJ/M respectively. This will be
described in more detail in later pages of this module, but the energy
of hydrolysis can be linked to another reaction (in this case the
transfer of a phosphate onto ADP to make ATP). In terms of
thermodynamics, these two reactions can be considered as individual
half reactions and can be combined into a single overall reaction such
that the favorable Free Energy of one "half reaction" can be used to
counteract the unfavorable Free Energy of another. In this case |
|
|
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
|
Cofactors/Cosubstrates |
ADP is a cosubstrate in glycolysis direction
and ATP is a coproduct |
|
|
|
||
|
DGo' |
-18.9 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the 3-Phosphoglycerate and ATP concentration would end up ~200 times higher than the product of the concetrations of 1,3bisPhosphoglycerrate and ADP. The Standard Free Energy favors ATP production - the reason for this is outlined above. |
|
Keq |
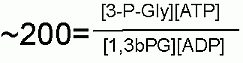 |
|
|
Comments |
For each glucose that started glycolysis
there are two glycerates. so 2 ATP are made for each of these
reactions. Our total ATP for glycolysis is now even. It took two ATP to
get the pathway started (1 for hexokinase, 1 for
phosphofructokinase-1). Now those two ATP have been returned. In terms
of what the organism needs most.. energy transfer from sugar to ATP we
are now even. |
|
|
"In cell" Substrate Concentrations* |
||
|
S1 = |
1,3 bisphosphoglycerate |
0.001 mM |
|
S2 = |
ADP |
0.14 mM |
|
P1 = |
3-Phosphoglycerate |
0.12 mM |
|
P2 = |
ATP |
1.85 mM |
|
DG for these conditions |
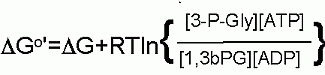 |
|
|
+0.1 kJ/M |
||
|
|
||
|
Mechanism for Chemistry |
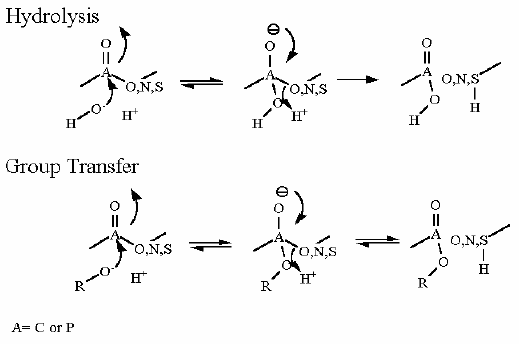 |
|
|
Mechanism for Enzyme |

|
|
|
Picture of Enzyme with substrate |
|
|
|
|
|
|
|
Phosphofructokinase
PFK-1 CHIME representation |
|
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.