 Glucose-6-Phosphatase Information
Glucose-6-Phosphatase Information Glucose-6-Phosphatase Information
Glucose-6-Phosphatase Information
|
Enzyme Name |
Glucose-6-Phosphatase |
|
|
Reaction Catalyzed |
Hydrolysis of phosphate from Glucose-6-Phosphate
|
|
|
Reaction Type |
Hydrolysis |
|
|
Rationale |
This enzyme is present maily in Liver cells. Recall that Liver is one of the primary places that stores glucose (as glycogen) and then releases it to the blood as required to maintain a constant blood glucose level. By removing the last phophate from glucose, it is now able to be released from the liver cells into the blood to be transported to other cells. Most cellular metabolites are charged. The reason is to maintain them inside the cell. uncharged molecules, like glucose for instance, are free to diffuse through the cell membrane. Remember the "core" of the membrane is hydrophobic. The last step in gluconeogenesis is to remove the last phosphate, in a hydrolysis. Hypothetically speaking - Why it just a hydrolysis. why not a group transfer to ADP to make more ATP? Think thermodynamics here... what is the DGo' for this hydrolysis reaction? and then what is the DGo' for synthesis of ATP from ADP + Pi. If these Standard Free Energies are added where does that leave us? |
|
|
Pathway involvement |
Gluconeogenesis ONLY |
Glucose-6-Phosphatase is not part of the glycolysis pathway. In glycolysis a separate enzyme (Hexokinase) adds a phosphate onto Glucose and is ATP dependent. The reaction catalyzed by Glucose-6-Phosphatase is a hydrolysis which in water is extremely difficult to reverse- because water is in very high concentration. |
|
Cofactors/Cosubstrates |
None |
|
|
DGo' |
-17 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the product concentrations would end up ~600 times higher than the substrates. This is a good way to end the gluconeogenesis pathway to ensure that glucose is produced in high enough concentration in the cell to be shipped out to the blood. Since bllod glucose concetration is supposed to be maintained at a fairly high level (100mg/dl = 5mM) then it is best if the liver cells can produce higher concentrations. |
|
Keq |
|
|
|
Comments |
Let's consider why hydrolysis reactions are very difficult to reverse in a water environment. First the DGo' greatly favors product formation (Glucose and phosphate in this case). But we also know that either very high product or very low substrate concentrations can make the observed Free Energy go the other way. What is always left of these calculations however is the concetration of water. Water concetration is pretty constant and very high - somewhere in the 45-50 M/l range. Water is in effect a substrate in a hydrolysis reaction. Its high concentration makes it difficult to reverse a hydrolysis reaction. These arguments do not necessarily apply in your Organic Chemistry course where reactions might be carried out in an organic solvent where water concentration is low. |
|
|
Substrate Concentrations* |
||
|
S1= |
Glucose-6-Phosphate |
0.001 mM |
|
S2= |
||
|
P1= |
Glucose |
5.0 mM |
|
P2= |
Pi |
1 mM |
|
DG for these conditions |
 |
|
|
-5 kJ/M |
||
|
Mechanism for Chemistry |
 |
|
|
Mechanism for Enzyme |
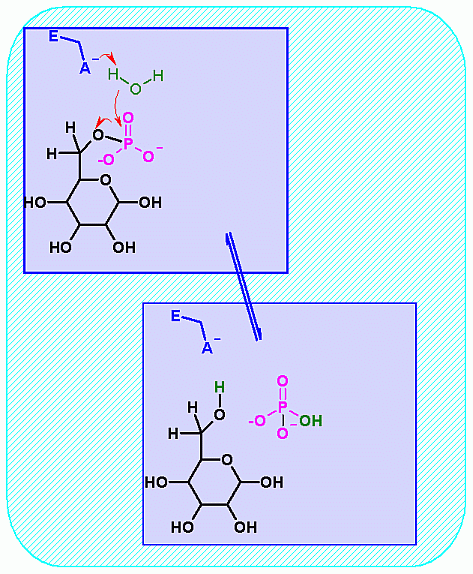
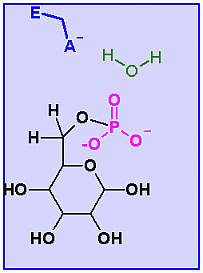 Glucose-6-Phosphatase. Animation of a Glucose-6-Phosphatase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Glucose-6-Phosphatase. Animation of a Glucose-6-Phosphatase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
In describing the reaction in the pictures at the right, an active site ASP near the phosphate on C6helps to ionize a water. The resulting HO- can then attack the phosphate to begin the hydrolysis. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. |
|
|
Pictures of Enzyme |
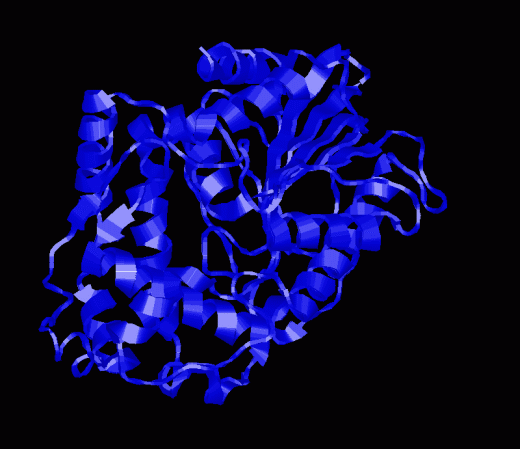 |
|
NOTE: Apparently there is no structure of a glucose-6-phosphatase. These pictures and the CHIME version below are from a glucose-1-phosphatase isolated from E. coli. While this is not the same enzyme it is liekly that the priciples apply.
|
||
|
Glucose-1-Phosphatase CHIME representaion | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.