Enzyme Name
Phosphoglucose Isomerase
Reaction Catalyzed
Isomerization of Glucose-6-P to Fructose-6-P

Reaction Type
Isomerization
Rationale
Isomerization to fructose. According to the rule of an aldol reaction the molecule must be set up in a certain fashion before an aldol cleavage can be affected. Glucose-6-phosphate (theoretically) IS correctly set to do this reaction. It does have an alcohol 2 C away from a ketone! BUT if we do an aldol cleavage NOW our products will be a 2 carbon piece and a 4 carbon piece. Our goal is two 3 carbon pieces.
If we do an isomerzation first then the ketone can move to C2. NOW we still an alcohol 2 carbons away from the ketone BUT the product of an aldol cleavage will be two 3 carbon pieces!
Pathway Involvement
Both Glycolysis and Gluconeogenesis
This reaction is freely reversible and in fact the STANDARD Free Energy of this reaction favors gluconeogenesis by a little bit. The observed reaction direction can easily be influenced by the substrate and product ratio (DG for these conditions below)
Cofactors/Cosubstrates
None
*Delta;Go'
+1.7 kJ/M
Starting from standard state and allowing the reaction to come to equilibrium the product (F6P) concentration would end up ~2 times LOWER than the substrate (G6P).
The Standard Free Energy favors glucose-6-phosphate production.
Keq
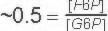
Comments
Notice from above that the STANDARD Free energy favors Substrate (G-6P) formation while in the cell the concentrations of Fructose-6P and Glucose-6P are such that the Free energy actually favors product (F-6P) formation.
"In cell" Substrate Concentrations*
S=
Glucose-6-Phosphate
0.083mM
P=
Fructose-6-phosphate
0.014 mM
ΔG for these conditions
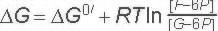
-2.7 kJ/M
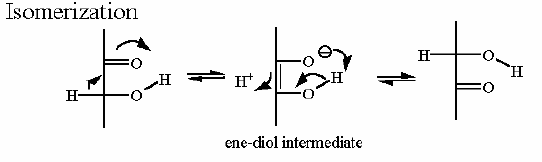
Mechanism for Chemistry
Mechanism for Enzyme

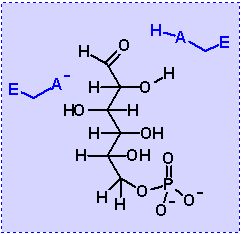 Phosphoglucose Isomerase. Animation of an Phosphoglucose Isomerase reaction Blue: represents the enzyme. The EA- and EAH represent the crucial enzyme active site amino acids in their basic (deprotonated) and acid (protonated) states, respectively. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Phosphoglucose Isomerase. Animation of an Phosphoglucose Isomerase reaction Blue: represents the enzyme. The EA- and EAH represent the crucial enzyme active site amino acids in their basic (deprotonated) and acid (protonated) states, respectively. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
In describing the reaction in the pictures at the right, it is initiated by the basic amino acid. This "pulls" a proton off C2 which frees a pair of electrons to swing up toward the carbonyl (C=O). one of the pairs in the C=O then "swings out" as it is attracted to the somewhat positive acidic amino acid on the enzyme. This is a classic "push -pull" method to get the reaction started.
Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above.
Picture of Enzyme with substrate
picture showing the subunit structure of phosphoglucose Isomerase. Only the main chain is shown represeted by these ribbons. The subunits are identical, one colored red the other blue. A substrate analog is shown in the active site as atom colored spheres
Active site + substrates
Same picture as above but the important atoms within 5A of the substrate analog are added as sticks.
Active site + substrates
Close up of the active site. The cartoons of the main chain atoms have been removed and only the atoms nearby the substrate analog are shown. In particular not the presence of the Glutamate and the Arginine at the top of the picture. These are acting as the base and acid, respectively from the shematic of the mechanism above.
|
Phosphoglucose Isomerase CHIME representation | |
