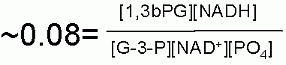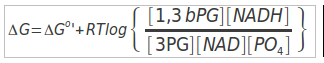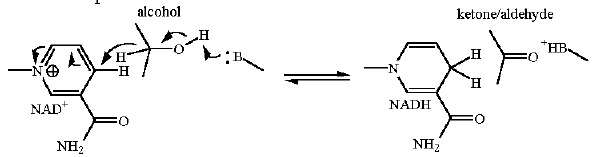|
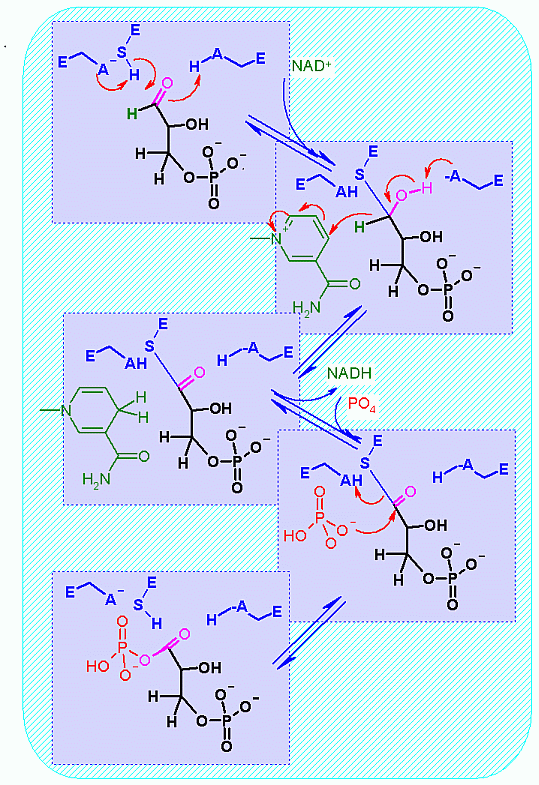
this enzyme demonstrates how acid, base and covalent calatysis ALL work together - not in isolation but in concert - to affect the reaction. (See the mechanism tab)
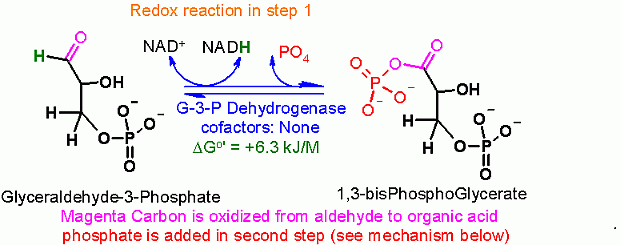
Oxidation of an aldehyde to an acid (combined with a one slight trick). The core of this reaction is the NAD+ dependent oxidation of the aldehyde in glyceraldehyde to an organic acid (glycerate).
The slight trick here is that it is actually converted to the phosphoanhydride rather than a free organic acid. This can be accomplished because of a covalently bound intermediate in the enzyme.
This is described in much more detail in the mechanism section below.
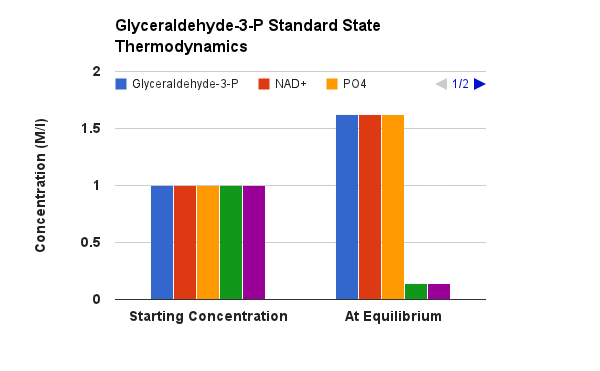
The justification for the twist lies in thermodynamics of the next enzyme catalyzed step of glycolysis. Remember, in one of the quiz questions, two modules ago, I indicated that the amount of energy released from hydrolysis of phosphate from an organic acid (phosphoanhydride) is MUCH higher than that for hydrolysis of a phosphate from an alcohol. This extra energy is indeed used in the next enzyme step to MAKE an ATP! (more on energy coupling later in this module).
Summing up - TWO ATP were needed to start the glycolysis pathway (hexokinase and phosphofructokinase-1) . NOW two ATP (remember there are two 3-phosphoglycerate for every glucose) are made in the
following reaction... we will be back where we started in number of ATP
|