Enzyme Name
Glyceraldehyde-3-Phosphate Dehydrogenase
Reaction Catalyzed
Written in the direction of glycolysis
- Oxidation of Glyceraldehyde-3-phosphate to glycerate-3-phosphate
- addtion of phosphate to C1
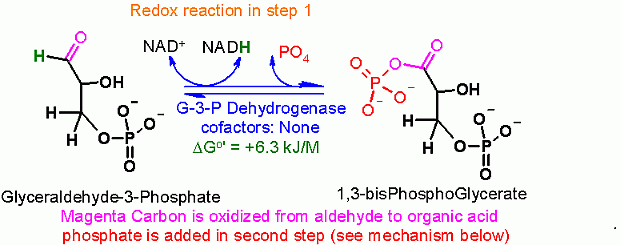
Reaction Type
- Oxidation/Redxution (REDOX)
- Hydrolysis type reaction (with a twist here since phosphate (instead of water) is used to attack it is called phosphorolysis)
Rationale
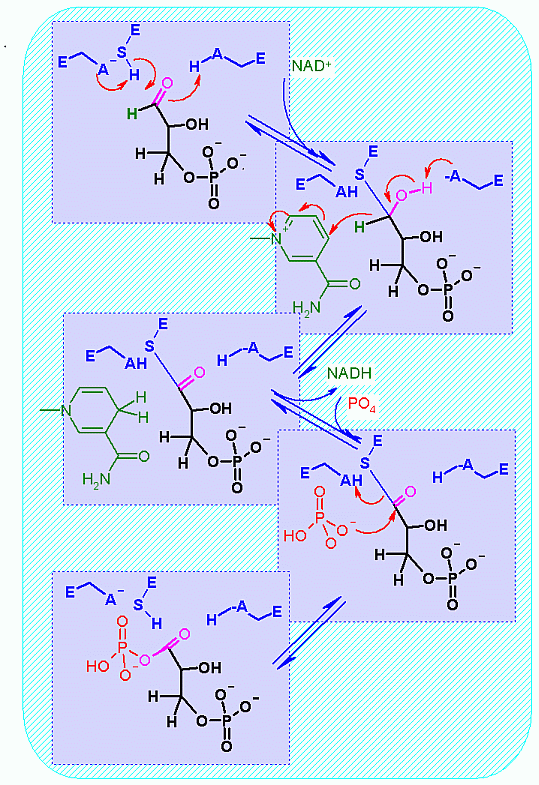
Oxidation of an aldehyde to an acid (combined with a one slight trick). The core of this reaction is the NAD+ dependent oxidation of the aldehyde in glyceraldehyde to an organic acid (glycerate).
The slight trick here is that it is actually converted to the phosphoanhydride rather than a free organic acid. This can be accomplished becuase of a covalently bound intermedicate in the enzyme. This is described in much more detail in the mechanism section below.
The justification for the twist lies in thermodynamics of the next enzyme catalyzed step of glycolysis. Remember, in one of the quiz questions, two modules ago, I indicated that the amount of energy released from phosphoanhydride hydrolysis is MUCH higher than that for hydrolysis of a phosphate from an hydroxyl group. This extra energy is used in the next step to MAKE an ATP! (more on energy coupling later in this module).
Summing up - TWO ATP were needed to start the glycolysis pathway (hexokinase and phosphofructokinase-1) . NOW two ATP (remember there are two 3-phosphoglycerate for every glucose) are made in the following reaction... we will be back where we started in number of ATP
Pathway Involvement
This reaction easily goes both directions depending on the concentrations of the reactants. (see discussion of ΔG below)
Cofactors/Cosubstrates
Gluconeogenesis cosubstrate: NADH; NAD+and PO4= are coproducts
ΔGo'
The Standard Free Energy favors Gluconeogenesis direction.
Keq
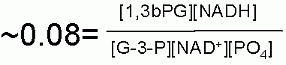
Comments
- Coupling of the phosphate addition to the NAD+ linked redox
- Formation of a required covalent enzyme bound intermediate (through a cysteine)
"In cell" Substrate Concentrations*
S1 =
S2 =
S3=
P1 =
P2 =
ΔG for these conditions
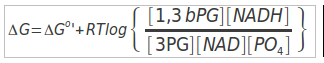
Mechanism for Chemistry step one
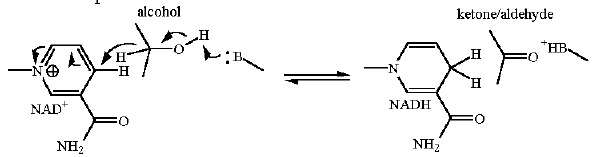
Complete Mechanism for Enzyme
- Ribbons Only the main chain is represented by these ribbons. There are four identical subunit
- one subunit Three of the subunits are deleted.
- as above with substrate Same picture as in "2" but and a substrate is added in. The atoms colored spheres. C=Gray; O=red; P=Orange.
- nearby AA AA near the subsrate are added as sticks
- NAD+ is added.
- Ribbons removed
- as above different orientation Active site AA + substarte + NAD but slightly different orientation to highlight the spatial orientation of the critical cysteine, NAD substrate and HIS
- C2 of NAD=magenta HIS=cyan exactly as above but the C4 of NAD is colored magenta (this is the carbon that picks up the hydride) and the HIS is colored cyan.
