|
Enzyme Name |
Aldolase | |
Reaction Catalyzed |
Aldol cleavage of Fructose-1,6-bisPhosphate AND Aldol Condensation of Dihydroxyacetonephosphate + glyceraldehyde-3-Phosphate
| |
Reaction Type |
Aldol Reaction | |
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
Cofactors/Cosubstrates |
In mammalian enzymes no cofactor or cosubstrates are required. In some bacterial enzymes a metal ion is required. | |
|
Rationale |
Aldol Reaction. I call this an "aldol reaction" because it goes BOTH directions; cutting into fragments is more commonly called an Aldol Cleavage, while putting two pieces together into one is called an Aldol Condensation. For this reaction the Standard Free Energy (ΔG°' - the thermodynamics tab) favors the formation of Fructose-1,6-bisphosphate. Are the two pieces identical? Not quite... how are they related? On one of the halves the ketone is on C2 while on the other it is on C1. How can they be made to look identical? Aldol Cleavage yields two different products. the subsequent Isomerization of one of the three carbon fragments makes them identical. If the aldol cleavage is performed on Fructose-1,6-bisphosphate, we will get two 3 carbon pieces each with a charged group (phosphate). The two halves DO each have a phosphate, so at least both halves contain the necessary charge groups to keep them in the cell. The glyceralhehyde does NOT NEED to do an ismomerization - this will continue along the glycolysis as is. ONLY dihydroxyacetone phosphate (ketone on C2) needs to be isomerized. Afterwards it is identical to the other half (glyceraldehyde-3-phosphate). The mammalian forms of this enzyme go through a required covalently bound substrate for catalysis. This is true regardless direction of the reaction. Meaning, both directions go through the same mechanism and utilize the same intermediate... as must be the case for all enzyme reactions. FOR ALL REACTIONS FROM HERE ON, THERE ARE TWO MOLECULES GOING THROUGH EACH CONVERSION FOR EACH GLUCOSE THAT STARTED. | |
|
ΔGo' |
+23.9 kJ/M |
 |
Keq |
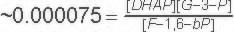 |
|
Comments |
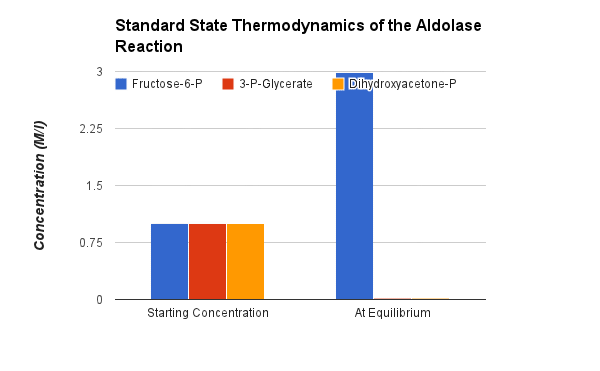
Starting from standard state and allowing the reaction to come to equilibrium the Fructose-1,6-bisphosphate concentration would end up ~15,000 times higher than the product of the concetrations of DHAP and G-3-P. The Standard Free Energy favors Fructose-1,6-bisphosphate production. Note: the Standard Free Energy greatly favors production of Fructose-1,6-bisphosphate.
| |
"In cell" Substrate Concentrations* |
||
S1 = |
Fructose-1,6-bisPhosphate | 0.031 mM |
S2 = |
||
P1 = |
Dihydroyacetonephosphate (DHAP) | 0.14 mM |
P2 = |
Glyceraldehyde-3-Phosphate (G-3-P) | 0.019 mM |
ΔG for these conditions |
 | -0.23 kJ/M |
Note this rather large turn around from ΔGo' to ΔG. This reaction does not have the same issues as that of a hydrolysis (water concentration at 50M/l) or that of an ATP dependent group transfer (ATP held fairly high and constant at 2mM). | ||
|
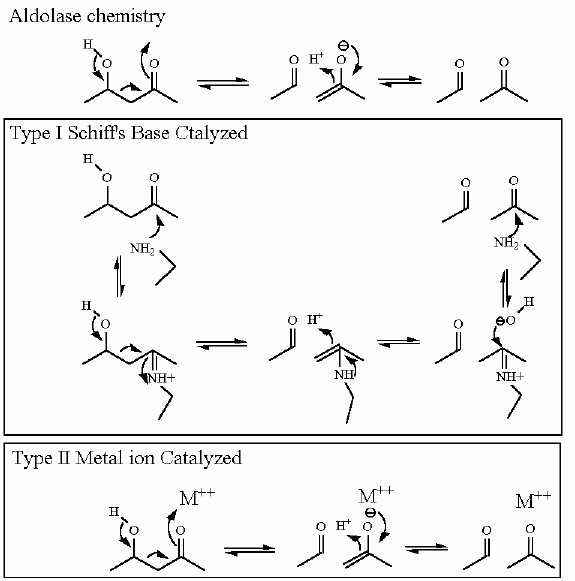
Mechanism for Chemistry |
 | |
Mechanism for Enzyme |
Hover your mouse over a step to reveal a description | |
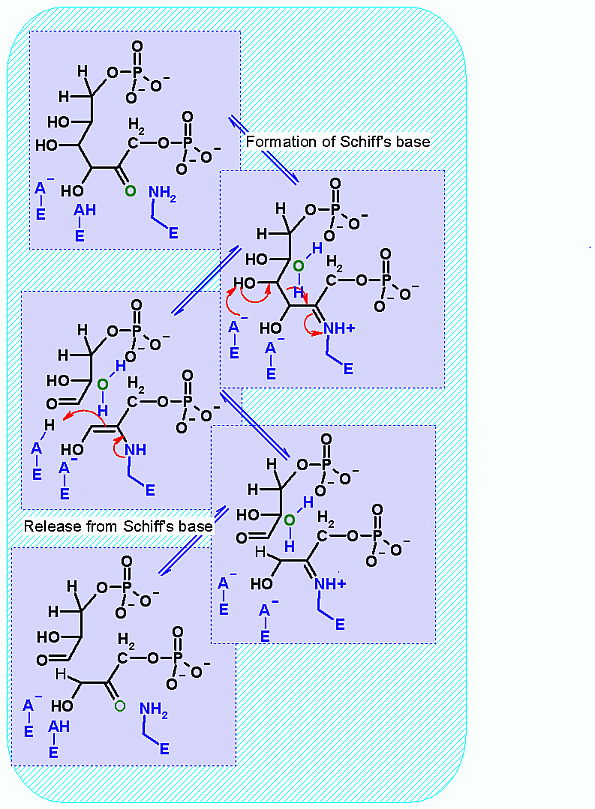
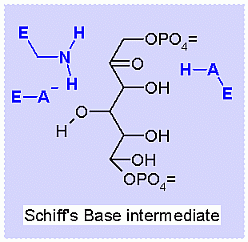 Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the aldol reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the aldol reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
This reaction happens in three phases: 1: Schiff's base formation; 2: Aldol Reaction; 3: Release of Schiff's base. These phases are labeled in the animation as well. The Schiff's base is formed to provide the necessary "pulling" force on the electrons to initiate the aldol reaction. Notice: how the "positive charge" on the nitrogen of the Schiff's base begins the process of the electron pulling cascade. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. |
||
|
Picture of Enzyme with substrate |
|
|
| ||
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |