 Aldolase Information
Aldolase Information Aldolase Information
Aldolase Information
Enzyme Name |
Aldolase | |
Reaction Catalyzed |
Aldol cleavage of Fructose-1,6-bisPhosphate AND Aldol Condensation of Dihydroxyacetonephosphate + glyceraldehyde-3-Phosphate
| |
Reaction Type |
Aldol Reaction | |
Rationale |
Aldol Reaction. I call this an aldol reaction because it goes BOTH directions; cutting into fragments is more commonly called an Aldol Cleavage, while putting two pieces together into one is called an Aldol Condensation. For this reaction the Standard Free Energy (DGo' - see below) favors the formation of Fructose-1,6-bisphosphate. Aldol Cleavage yields two differnt products. the subsequent Isomerization of one of the three carbon fragments makes them identical. If the aldol cleavage is performed on Fructose-1,6-bisphosphate, we will get two 3 carbon pieces each with a charged group (phosphate). Are the two pieces identical? Not quite... how are they related? The two halves to DO each have a phosphate, what do you have to do to ONE of the halves to get the phosphates on top of each other (superimpose). The pieces from the aldol reaction are not identical, but are related to each other by a simple conversion. Another isomerization! On one of the halves the ketone is on C2 while on the other it is on C1. How can they be made to look identical? The glyceralhehyde does NOT NEED to do an ismomerization. ONLY dihydroxyacetone phosphate (ketone on C2) needs to be isomerized. Afterwards it is identical to the other half (glyceraldehyde-3-phosphate). FOR ALL REACTIONS FROM HERE ON, THERE ARE TWO MOLECULES GOING THROUGH EACH CONVERSION FOR EACH GLUCOSE THAT STARTED. | |
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
Cofactors/Cosubstrates |
In mammalian enzymes no cofactor or cosubstrates are required. In some bacterial enzymes a metal ion is required. | |
DGo' |
+23.9 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the Fructose-1,6-bisphosphate concentration would end up ~15,000 times higher than the product of the concetrations of DHAP and G-3-P. The Standard Free Energy favors Fructose-1,6-bisphosphate production. |
Keq |
 |
|
Comments |
Note: the Standard Free Energy grealy favors production of Fructose-1,6-bisphosphate. The mammalian forms of this enzyme go through a required covalently bound substrate for catalysis. This is true regardless direction of the reaction. Meaning, both directions go through the same mechanism and utilize the same intermediate... as must be the case for all enzyme recations.
| |
"In cell" Substrate Concentrations* |
||
S1 = |
Fructose-1,6-bisPhosphate | 0.031 mM |
S2 = |
||
P1 = |
Dihydroyacetonephosphate (DHAP) | 0.14 mM |
P2 = |
Glyceraldehyde-3-Phosphate (G-3-P) |
0.019 mM |
DG for these conditions |
 | |
-0.23 kJ/M | ||
Note this rather large turn around from DGo' to DG. This reaction does not have the same issues as that of a hydrolysis (water concentration at 50M/l)or that of an ATP dependent group transfer (ATP held fairly high and constant at 5mM). | ||
Mechanism for Chemistry |
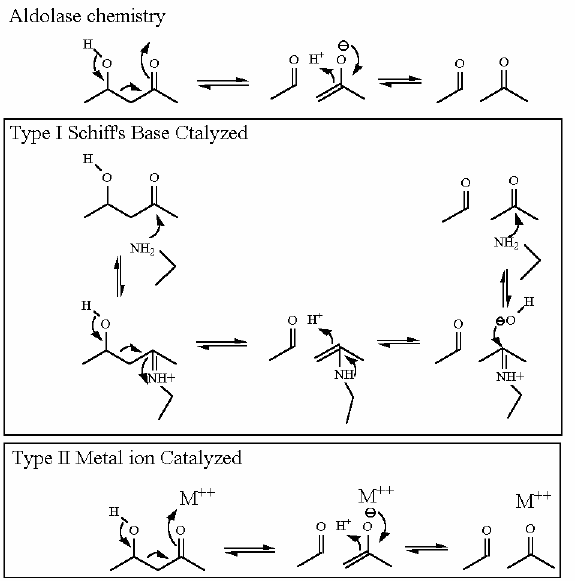 | |
Mechanism for Enzyme |
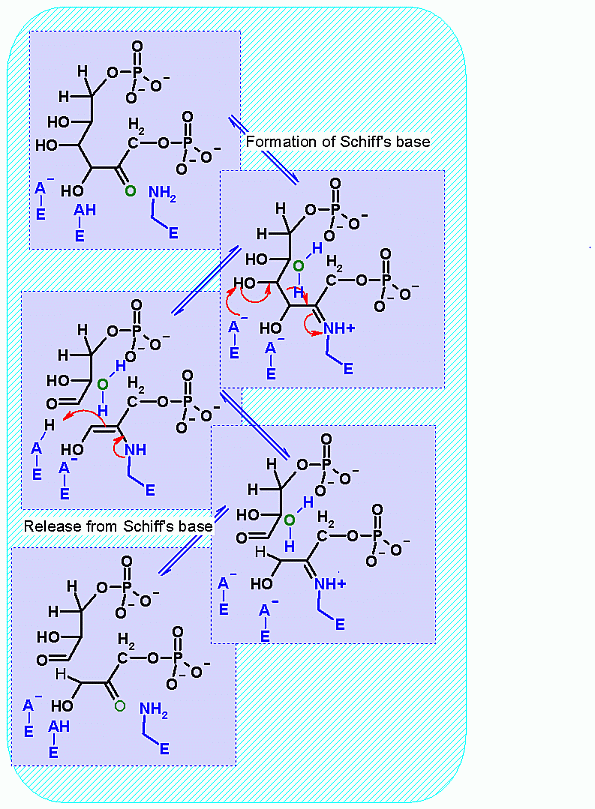 | |
 Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Aldolase. Animation of the Aldolase reaction Blue: represents the enzyme. The E-NH2 represents the crucial enzyme active site amino Lysine in their basic (deprotonated). "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
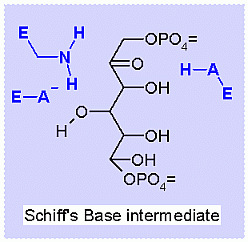
This reaction happens in three phases: 1: Schiff's base formation; 2: Aldol Reaction; 3: Release of Schiff's base. These phases are labeled in the animation as well. The Schiff's base is formed to provide the necessary "pulling" force on the electrons to initiate the aldol reaction. Notice: how the "positive charge" on the nitrogen of the Schiff's base begins the process of teh electron pulling cascade. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. |
||
Picture of Enzyme with substrate |
|
|
| ||
|
Phosphofructokinase PFK-1 CHIME representation | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.