 Phosphofructokinase Information
Phosphofructokinase Information Phosphofructokinase Information
Phosphofructokinase Information
Enzyme Name |
Phosphofructokinase (PFK-1) | |
Reaction Catalyzed |
ATP Dependent Phosphorylation of C1 of Fructose-6-Phosphate
| |
Reaction Type |
Group Transfer | |
Rationale |
Why is another phosphate transferred to Fructose? A Second ATP dependent group transfer. After the isomerization can we do a group transfer at C1? Why or could we not transfer a phosphate to C1 of glucose? Why do we need to do transfer another phosphate at all? Think about the two pieces after an aldol cleavage. If only one phosphate were on fructose, Would both fragments be charged? If both fragments are not charged, then what would happen to the uncharged one? | |
Pathway Involvement |
Glycolysis ONLY |
PhosphoFructokinase (PFK-1) is not part of the gluconeogenesis pathway. In gluconeogenesis a separate enzyme (Fructose-1,6-bisphosphatase) hydrolyzes a phosphate from C1 Fructose-1,6-bisphosphate and is not ATP dependent. Rather it performs a simple hydrolysis reaction to cleave off phosphate. |
Cofactors/Cosubstrates |
ATP is a cosubstrate; ADP is a coproduct | |
DGo' |
-14.2 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the products (F-1,6-bP and ADP) concentration would end up ~300 times higher than the substrates (F6P + ATP). The Standard Free Energy favors Fructose-1,6-bisphosphate production. |
Keq |
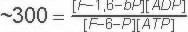 |
|
Comments |
Why is ATP required? What would the DGo' for putting a phosphate on glucose be if we used inorganic phosphate (reversal of a hydrolysis reaction) instead of ATP? It would be +15 kJ/M. Which direction would be favored then? There are four identical subunits in the active enzyme. This enzyme is PFK-1. There is another phosphofructokinase (PFK-2) that is not involved in the metabolic pathway. This a a separate enzyme that transfers a phosphate to C2 of Fructose-6-phosphate. to produce Fructose-2,6-bisphosphate. this compound is not metabolized, but rather serves ONLY a regulatory function. We will learn much more about this enzyme and the role of fructose-2,6-bisphosphate in Module 8. This is an allosterically (literally means another site) regulated enzyme. Onr of the key regulation points between glycolysis and gluconeogenesis. MUCH more about this in moule 8. For now just bear in mind that the kinetic properties of this enzyme can be greatly altered by compounds binding to a site that is well removed from the active in the protein structure. | |
"In cell" Substrate Concentrations* |
||
S1 = |
Fructose-6-Phosphate | 0.014 mM |
S2 = |
ATP |
2 mM |
P1 = |
Fructose-1,6-bisPhosphate | 0.031 mM |
P2 = |
ADP |
0.14 mM |
DG for these conditions |
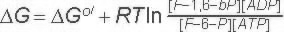 | |
-18.8 kJ/M | ||
Mechanism for Chemistry |
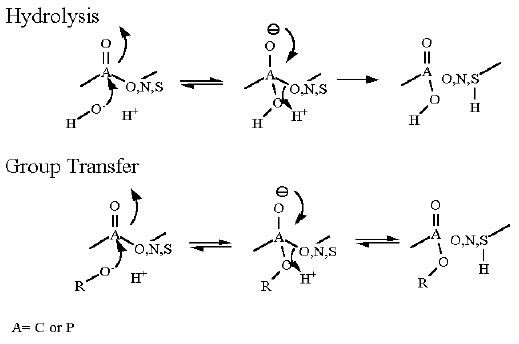 | |
Mechanism for Enzyme |
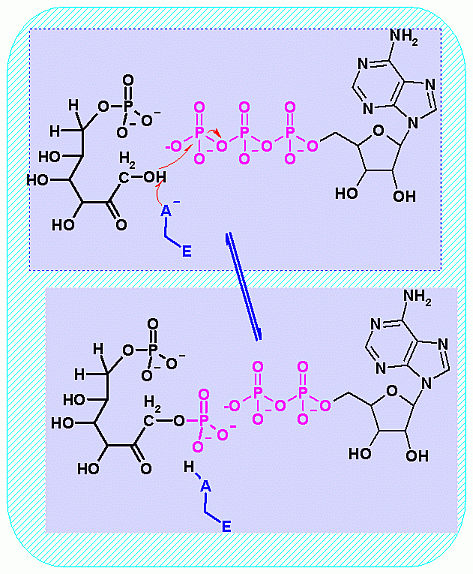
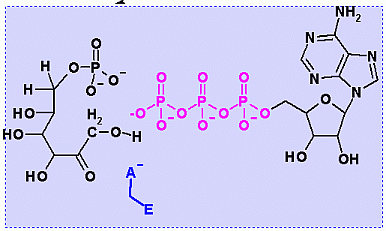 Phosphofructokinase PFK-1. Animation of the PFK-1 reaction Blue: represents the enzyme. The EA- represent the crucial enzyme active site amino acids in their basic (deprotonated) . "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Phosphofructokinase PFK-1. Animation of the PFK-1 reaction Blue: represents the enzyme. The EA- represent the crucial enzyme active site amino acids in their basic (deprotonated) . "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
In describing the reaction in the pictures at the right, it is initiated by the basic amino acid. This "pulls" a proton off C1 which frees a pair of electrons to swing up toward the alcohol (C-OH). The resulting C-O- then "swings out" to attack phosphate group. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. | |
Picture of Enzyme with substrate |
In the most active form of this enzyme ADP is bound in two places: at the active site (cyan) and at the allosteric regulation (white) site. Fuctose-6-phosphate (yellow) is also bound at the active site. Not shown in this picture, PFK1 is a tetramer in its native state. The allosteric ADP binding site is very close to the subunit interface. The alloteric site is very close to the active site on the another subunit. | |
Active site + substrates |
The amino acids at the active site are highlighted as well as the substrates ADP and fructose-1,6-bisphophate. Not shown in these pictures is a critical arginine from the neighoring subunit that makes an important interaction with a phosphate group. | |
Active site + substrates |
Closeup of the active site. Note the Aspartate that is in alignment between the C1 fructose and the ADP. This is the base that will catalyze the reaction. There are several arginines near phosphate groups to provide important binding sites. One arginine is not shown. The one that comes from a neighboring subunit. | |
|
Phosphofructokinase PFK-1 CHIME representation | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.