Repsonse to Organisms Physiology
These same pathways not only respond the cells energy state but also to the organisms energy state. While the END result
is similar.... some change that alters the equilibrium between GOOD and POOR catalytic structures, the mechanism must be (and is)
entirely different. The organism measures its energy state not in terms of [ATP] but in blood glucose concentration. When blood
glucose is high, the organism should take up glucose into the liver and store it as glycogen. On the other hand as blood glucose
decreases, the organism should break down glycogen AND increase the rates of gluconeogenesis in the liver to for highest rates of
glucose synthesis and ship it out to the blood. In this way the blood glucose levels remain fairly constant throughout the day so
long as there is glycogen to break down.
The pancreas reacts to blood glucose as follows:
- in response to HIGH blood [glucose] it secretes insulin. => take up glucose and synthesize glycogen
- in response to LOW blood [glucose] it secretes glucagon. => break down glycogen and increase gluconneogenesis; ship glucose
to blood
Let's take a closer look at the glucagon reponse in the liver.
In short many enzymes in liver cells are covalently modified with a phosphate group as [glucagon] increases in the blood.
It occurs via a cascade type system that is somewhat reminiscient of the blood coagulation cascade that we have looked at on
occasion.
Enzymes that are covalently modified with a phosphate group:
- phosphofructokinase-2/Fructose-2,6-bisphosphatase (single peptide that contains two enzyme active sites: PFK-2 and
F26BPase)
- synthase/phosphorylase kinase
- Glycogen synthase
- Glycogen phosphorylase
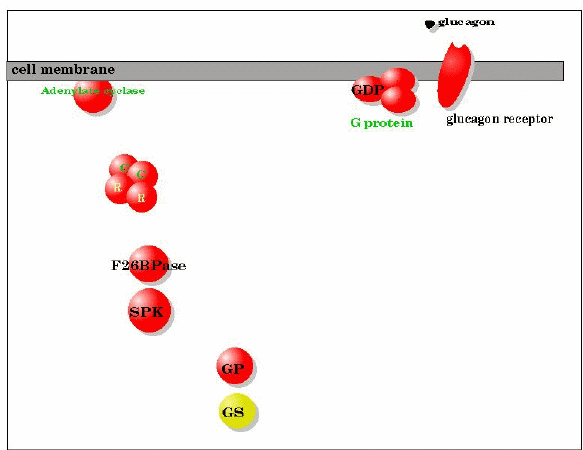
Key:
| Colors: | Abbreviations: |
| Active Enzyme | G protein: GDP binding protein |
| Inactive enzyme | F26BPase: Fructose 2,6 bisphosphatase |
| Phosphorylated (label color) | SPK: Synthase/Phosphorylase Kinase (ATP
dependent phosphorylation of glycogen synthase and glycogen phosphorylase) |
| Not
Phosphorylated (label color) | GS:
glycogen synthase |
| Non Proteins | GP: glycogen phosphorylase |
| Protein never modified (label color) | R: regulatory subunit of cAMP
dependent protein kinase |
| C: catalytic subunit of cAMP dependent protein kinase |
 Short description of the glucagon "activation" process:
Short description of the glucagon "activation" process:
- Glucagon in the blood is secreted by the pancreas in response to LOW blood sugar
- Glucagon binds to the glucagon receptor. The receptor uses ATP to phosphorylate itself (autophophorylation)
- G protein, in contact with phosphorylated receptor exchanges GTP for GDP
- It's subunits separate into one containing the GTP activity and two others
- The GTP containing subunit of the G protein contacts Adenylate cyclase. Adenylate cylase starts to produce cAMP.
- As the [cAMP] in the cell rises it binds to cAMP dependent protein kinase ( atetrameric protein containing two
catalytic subunits and two regulatory subunits) as a tetramer it is inactive.
- cAMP binding to cAMP dependent protein kinase causes the regulatory subunits to dissociate from the catalytic subunits. The
catalytic subunits are now active for ATP dependet phosphorylation.
- The active cAMP dep protein kinase phophorylates (among others) two proteins: 1 synthase/phosphorylase kinase, 2 the peptide that
contains phosphofructokinase-2 and frucose-2,6-bisphosphatase activities.
- The protein # 2 in te prvious step is nost active in the fructose 2,6-bisphosphatase activity it starts to hydrolyze
fructose-2,6-bisphosphate to fructose-6-phosphate. The synthase/phosphorylase kinase is now active to phosphorylate glycogen
synthase (least active form) and glycogen phophorylase (most active form)
- The activities of glycogen synthase and glycogen phosphorylase ensure that glycogen is mostly broken down under these cicumstances
Short description of the "relaxation" back to "normal" process:
- Glucagon concentration decreases in blood (It is a small peptide with a short half life in the blood)
- Glucagon dissociates from the recpetor. The receptor become conformation is such that it stop autophosphorylation.
- The GTP bound protein has a SLOW inheren GTP hydrolysis activity so with some time it hydrolyzes GTP to GDP +
PO4=
- The G protein reassociates and this inactivates the adenylate cyclase activity
- The enzyme phosphodiesterase hydrolyzes cAMP to AMP
- The GENERAL enzyme, protein phosphatase, hydrolyzes the phosphate groups off of most phosphorylated proteins.

 Short description of the glucagon "activation" process:
Short description of the glucagon "activation" process: