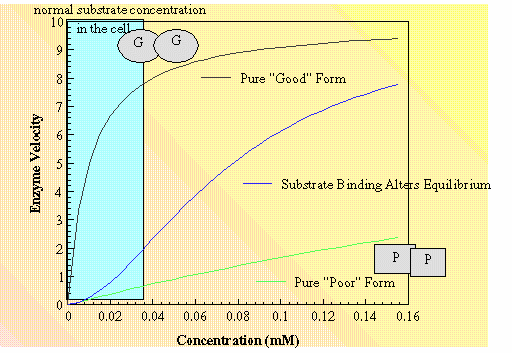
The enzyme exists in two slightly different structures. In the case above one structure catalyzes the reaction effiently even at low substrate concentrations (Good form). If both subunits of the enzyme are "locked" into this form then the "normal Michaelis-Menten kinetics are observed with a definable kcat and Km. The other conformation has rather poor catalytic characteristics (Poor form).... in this case the same kcat but a much higher Km. If the enzymes were "locked" into this conformation the normal Micaelis-Menten kinetics are observed with definable kcat and Km. IF on the other hand the enzyme switches conformations at an observable rate then a curve like the middle one is observed. In the substrate free state the equilibrium between Poor and Good states favors the Poor state. Substrate binds preferentially to the Good state and the equilibrium between the two conformations tips in that direction. Allosteric effectors bind to the enzyme at a site remotte from the enzymes active site. They also effect the observed equilibrium. Allosteric activators tip the equilibrium toward the Good state Allosteric inhibitors tip the equilibrium toward the Poor state.