|
Enzyme Name |
Complex V |
|
| |
||
Reaction Catalyzed |
hdrolysis (reverse)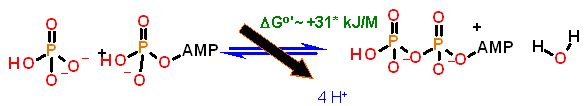
|
|
Reaction Type |
Hydrolysis | |
| Pathway Involvement
|
Oxidative Phosphorylation |
|
| Cofactors/Cosubstrates
|
none | |
|
Rationale |
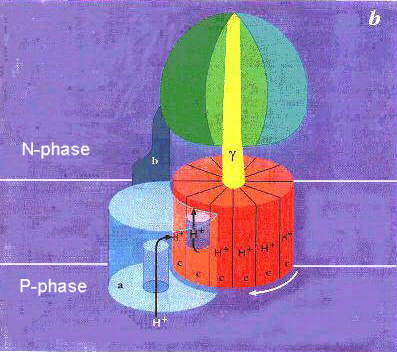
The official database for keeping protein structures also has a Molecule of the month page. This one addresses complex V or F1F0 ATPase. The complete structure has been solved. It is an extremely complex protein containing many subunits and a mechanical aspect to its function. There are two separate - but - coupled reactions: H+ gradient pumping and ATP synthesis. This reaction is written as proceeding toward ATP synthesis which has an extrememly unfavorable standard free energy by itself. BUT since this coupled to H+ gradient release, it too must be taken into account. The H+ gradient has sufficient energy to enzure that this is now a reverisble reaction. Note that I indicated that it is now a reversible reaction. This means that indeed it can hydrolyze ATP to generate a H+ gradient too. The object of all the other systems (glycolysis, citric acid cycle, electron transport) is to keep the H+ gradient energy just higher than that requird to synthesize ATP A demonstration with the rotating portion of the enzyme (the F1 part) was the first really cool result from single molecule studies with fluorescence. A molecule that glows brightly was attached to another long straight molecule - which in turn was attached to the ATPase. Under a microscope then you can actually watch this rotation. While it does not shed much light on the mechanism - it is fascinating to watch. Check out the animation on the "mechanism" section. It tried to wrap the essential items regarding proton gradient, mechanical action and ATP synthesis all together. |
|
| |
||
|
ΔGo' |
+31 kJ/M (NOTE: This does not take any H+ gradient into account) | Starting from standard state (all concentrations at 1 M/l) and allowing the reaction to come to equilibrium the substrates ADP and PO4= concentration would end up |
| The Standard Free Energy favors ADP. This should be no surprise. |
||
| Comments |
The Standard Free Energy does not really provide the whole story for this enzyme. - reason - as pointed out above, it does not take into account the energy behind the H+ gradient. This enzyme is made to couple that energy to the formation of ATP. If taken out of the membrane (so that there is no gradient energy to utilize, this enzyme becomes very effective at hydrolyzing ATP to ADP and phosphate.
The ΔG°' for this reaction as stated above only takes the chemistry into account. This is actually not the case. Since the H+ gradient generation is linked to enzyme function (so long as the membrane is intact), this too must be taken into account. The ΔGtotal = The ΔGchemistry + The ΔGH+grad ΔGchemistry= of the chemistry also taking into account the [ATP] and [ADP]. ΔGH+grad= the ΔG due to the proton gradient |
|
| ΔG in a typical mitochondrion |
||
| varies but is usually just less than 0 kJ/M | ||
|
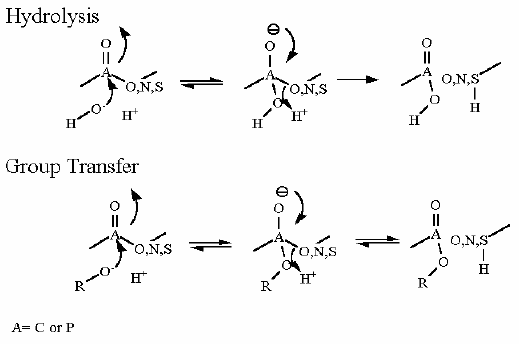
Mechanism for Chemistry |
 |
|
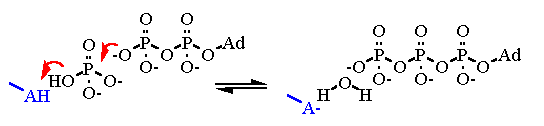 The above reaction mechanism Shows the basics about the bond formation on the ATPase active site. The real problem here is that the thermodynamics lies far to the left. Energy must be input into the system to make it proceed to the right. That energy is input after the bond formation to make ATP release from the active site. This is caused by a series of conformational changes in the protein. That energy for the conformational changes are derived from a H+ gradient across the membrane. The energy to setup the H+ gradient is derived from electron transport. | ||
|
Note: this IS a reversible reaction. Should the proton gradient have less ΔG than that for ATP hydrolysis - ATP hydrolysis can be used to form the proton gradient!! |
||
| Pictures of Enzyme with substrate |
 |
|
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |
