|
Enzyme Name |
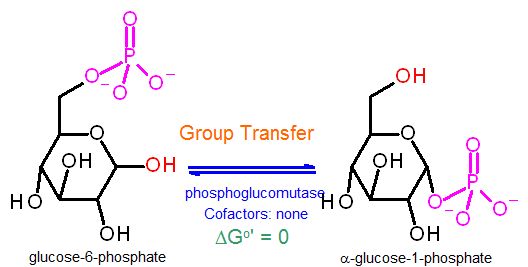
Phosphoglucomutase | |
Reaction Catalyzed |
Group Transfer reaction to move the phosphate group between C1 and C6 of glucose
| |
Reaction Type |
Group Transfer Reaction | |
Pathway Involvement |
Glycogen Synthesis AND Breakdown |
|
Cofactors/Cosubstrates |
None | |
|
Rationale |
Why is this Group Transfer chemistry? Afterall glucose-6-phosphate and glucose-1-phosphate are isomers. Doesn't that make this an "isomerization"? NO - because to a biochemist the isomerization reaction specifically means the "swapping" of adjacent alcohol and carbonyl (ketone/aldehyde). Here a phosphate is being moved from C6 to C1 (and back) by chemistry that is defined as group transfer (the "phosphate group" is transferred). In order to make glycogen one must attach C1 of a glucose to the C4 of the terminal glucose on the existing glcyogen polymer. Ultimately this end result is a group transfer reaction (transferring a glucose to the polymer). IFFFF we were to proceed with to building the polymer directly with glucose-6-phosphate it would actually be a "reverse hydrolysis" (condensation) as we are trying to "attack" an -OH group (on C1 glucose) with an -OH group (from the C4 polymer) - producing water as a byproduct. We already know that condensation reactions are thermodynamically very difficult in water (living cells). Difficult to the point of being impractical! This enzyme represents the first of a two step process to make the polymerization reaction more favorable thermodynamically. In this step we are simply moving the phosphate already in place on C6 to the C1 position. NOW the polymerization reaction is a group transfer as PO4= is the second product of the polymerization not water. So we are there... right? There is one last issue to consider. The thermodynamics of this particular reaction have a ΔG°' ≈ 0 (Keq=1). If it were up to this enzyme alone, only about half of the glucose-6-phosphate would be converted. While this is not awful, it's not great either. So, when polymerizing, we need something to dras the reaction toward G-1-P. the next step in the pathway uses TWO ATP equivalents to give it very favorable thermodynamics. Since these two are connected in the pathway - this one will proceed forward - as the next enzyme "pulls" the glucose-1-phosphate to the next product. What i negelected to add in the previous paragraph is that of course this enzyme "swings both ways" - meaning it plays a part in both glycogen synthesis AND glycogen breakdown. It goes which ever way the [product]/[substrate] ratio "tells" it to. How do we know? It tunrns out that we will find in module 8 that synthesis and breakdown rates are heavily regulated. the dominant direction is determined by kinetics of the enzymes glycogen synthase and glycogen phosphorylase. We will cover this in gory detail in a couple weeks. Stereochemistry of C1: Notice the stereochemistry of C1 in glucose-6-phosphate it indeterminate (it is neither up nor down) but in α glucose-1-phosphate, the bond is pointing down to the "D" position. Recall that so long as C1 only has an -OH it is not solely in a 6 member ring as shown, but rather is opening and closing rapidly and randomly. When actually in a six member ring it is about half the time "D" and half the time "L". On the other hand as soon as the phosphate is put on, it must stay a closed six member ring with well-defined stereochemistry in this case in the "D" position. Just ot make things confusing, this is termed "α". | |
|
ΔG°' |
≈0 kJ/M | |
Keq |
[G-1-P]/[G-6-P] ≈ 1 | |
Comments |
Starting from standard state and allowing the reaction to come to equilibrium the concentrations of glucose-6-phosphate and glucose-1-phosphate would end up about equal. The Standard Free Energy does not favor production of either compound. | |
|
Mechanism for Chemistry |
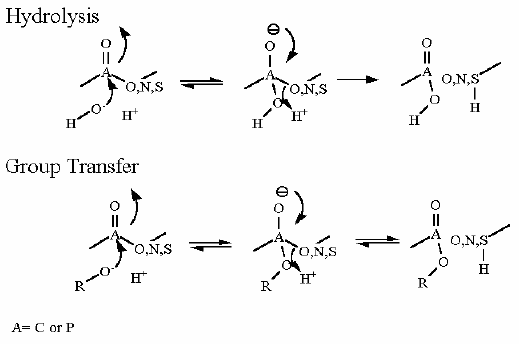 | |
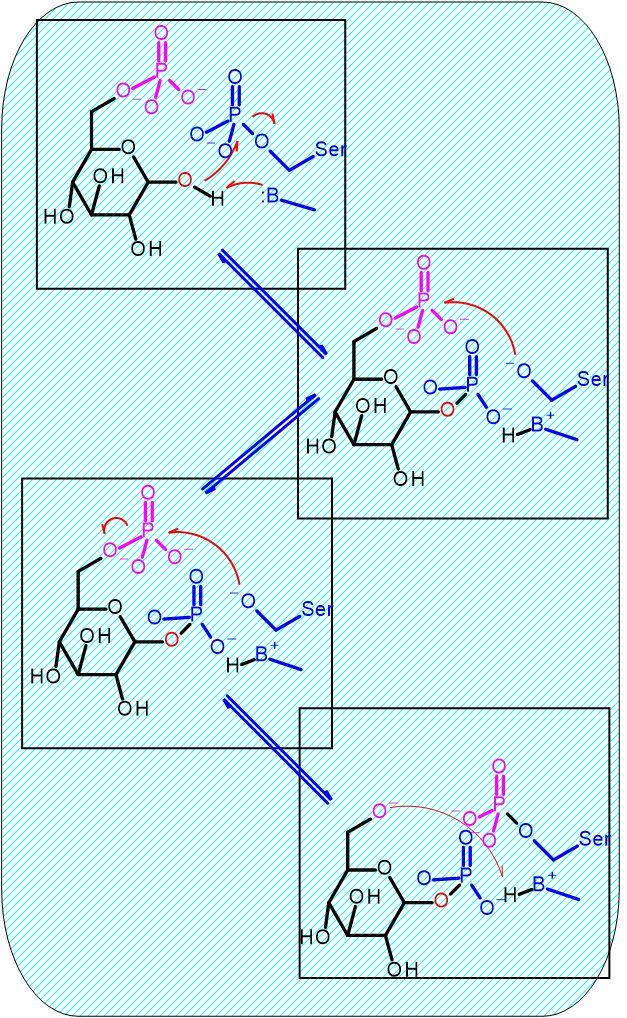
Mechanism for Enzyme Note: the enzyme must be phophorylated to start |
Hover your mouse over a step to reveal a description | |
|
Picture of Enzyme with substrate |
 |
|
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |