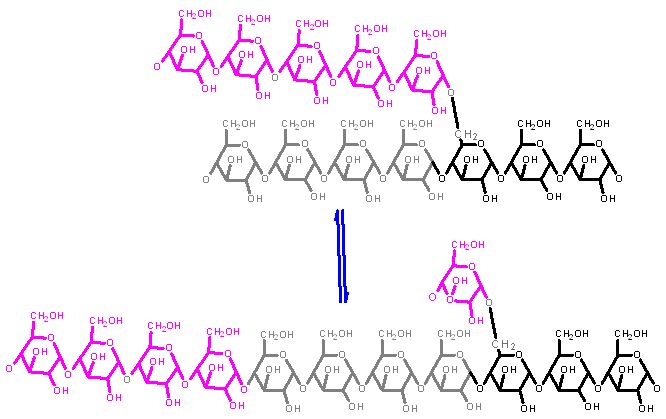
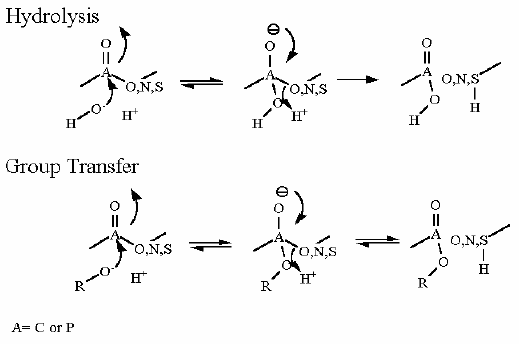
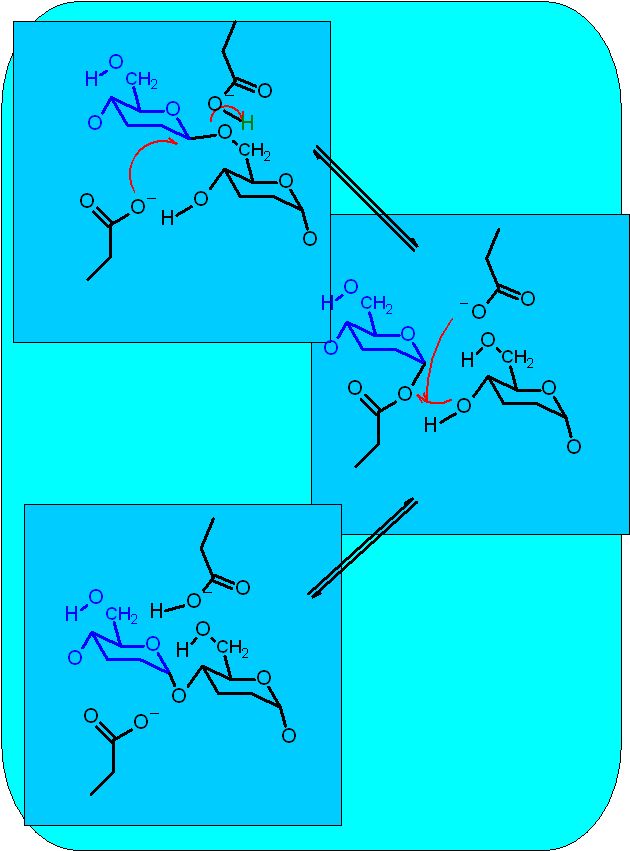
This is part of the pathway that breaks down glycogen. It works in concert with the next enzyme of the pathway - glycogen phosphorylase. That enzyme can only pick off one glucose at a time AND only if it is linked via a 1→4 bond. Eventually, as this enzyme approaches a branch point it cannot proceed further. The debranching enzyme then moves a segment to the adjacent polymer section.
As with the branching enzyme the segment appears from this 2D picture as if it is moving a long distance. This is likely not the case, but rather the C4 of the end is nearby in space to the C4 of the nearby branch because of the helical nature of the polymer.
Notice that one glucose is left behind on the branch point. This appears not to help much... it must be hydrolyzed (use water to cleave off) off by yet another enzyme, glucose 1→6 glycosidase. Whose sole function appears to be to clean up the leftover branches.What do you think the next step in metabolism of this single glucose is - If this happens in liver cells there are two possible fates.
|