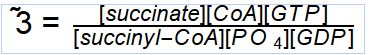Enzyme Name |
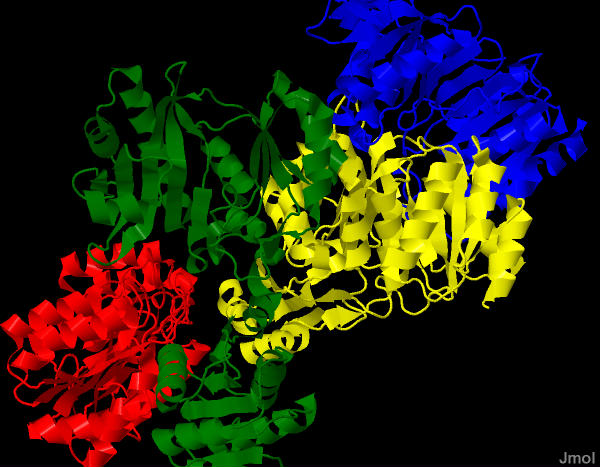
Succinyl-CoA Synthetase |
|
| |
||
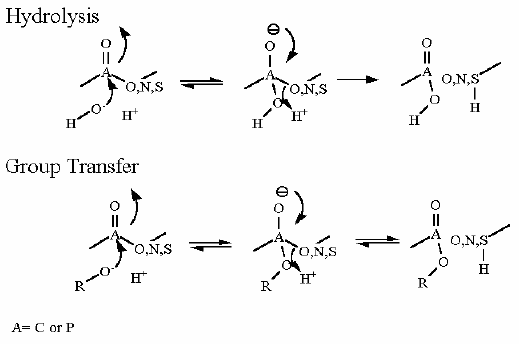
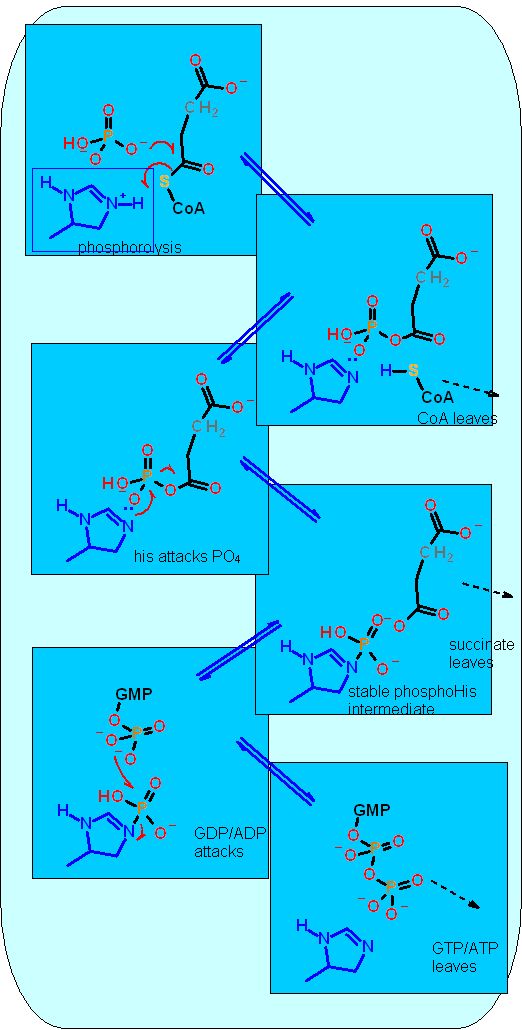
Reaction Catalyzed |
two step reaction:
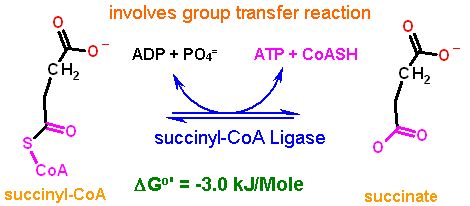
|
|
| Reaction Type |
Two Step Reaction
|
|
| Pathway Involvement | Citric Acid Cycle
|
|
| Cofactors/Cosubstrates | The ADP "equivalent" GDP and PO4= (phosphate) are co-substrates and the ATP "equivalent" GTP and coenzyme A are co-products. no other cofactors are required | |