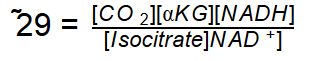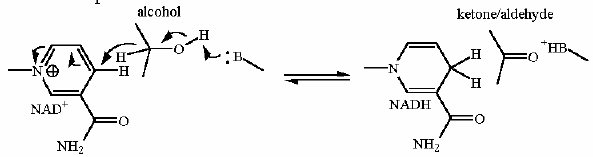|
Enzyme Name |
Isocitrate Dehydrogenase |
|
| |
||
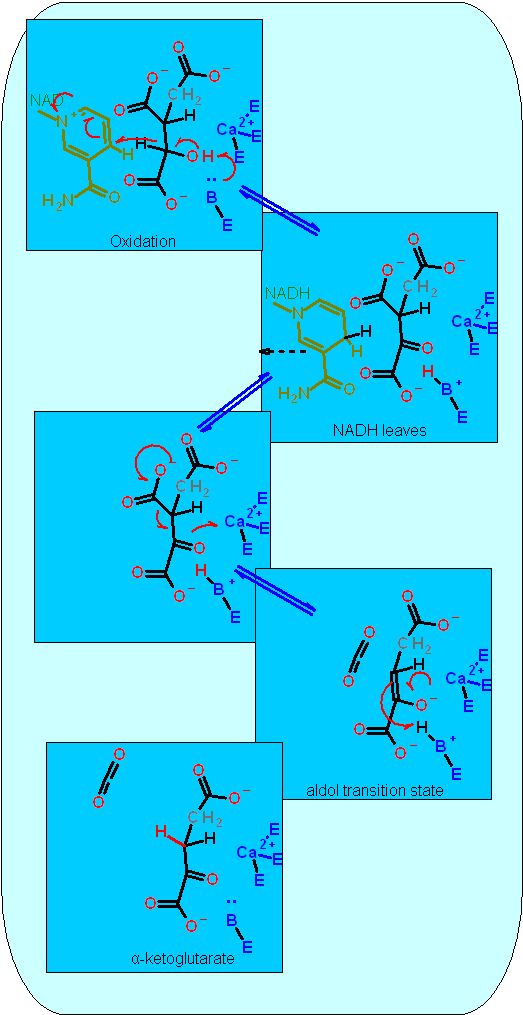
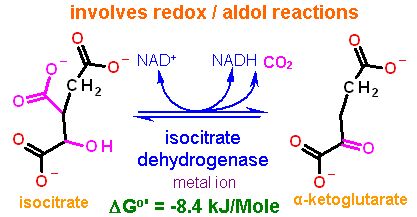
Reaction Catalyzed |
two step reaction:
|
|
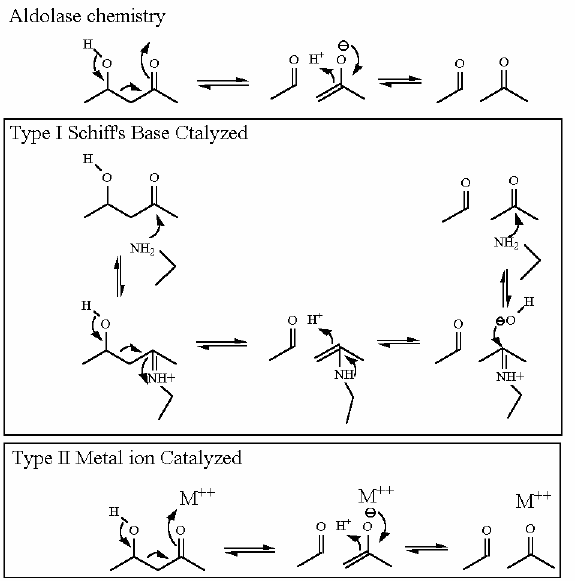
Reaction Type |
Two Step Reaction
|
|
| Pathway Involvement |
Citric Acid Cycle |
|
| Cofactors/Cosubstrates |
Requires NAD+ as a cosubstrate for the oxidation and a metal ion (frequently magnesium) to aid the aldol reaction. | |