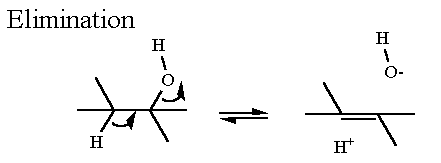|
There are two essential features to the citric acid cycle.
- Fully oxidize two carbons to carbon dioxide. DONE.
- Return the initial starting material (oxaloacetate). Middle step.
Oxaloacetate contains four carbons - two of which are carboxylic acids and one of which is a ketone. After forming citrate (six carbons) and removing two carbons we are back to four carbons (check). Of these four carbons two are carboxylic acids (check) but neither of the other two even HAVE a carbon oxygen bond anywhere.
The problem remains - how to form the ketone. You cannot replace a C-H bond with a C-OH bond. we do not have a chemsitry (of the five) for that. consequently there are three necessary reactions
- Desaturation (oxidation) of a C-C bond (to form a C=C bond)
- Hydration of a C=C bond (to form an alcohol)
- Oxidation of the alcohol to a ketone.
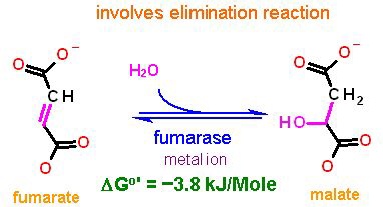
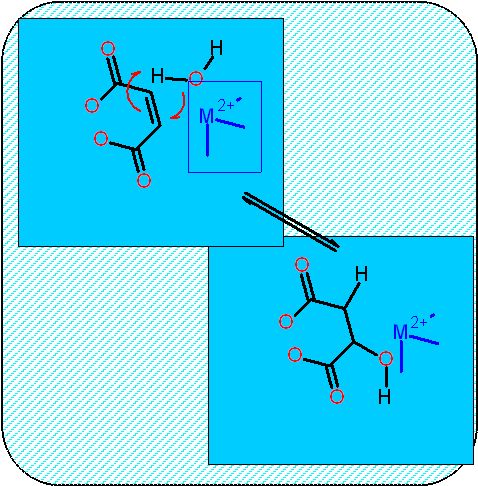
This is the middle of these three. An elimination reaction highly similar to enolase of the glycolysis pathway - here though there is not phosphate involved.
|