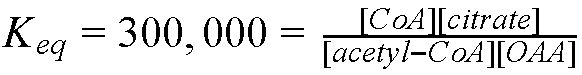|
Before beginning the citric acid cycle, there are four carbons remaining of the 6 from the original glucose.
- glucose was split into two 3-carbon compounds
- Each of those 3-carbon compounds was converted into acetyl-CoA (2-carbons each) with the third being removed as carbon dioxide.
The goal is to completely oxidize all 6 carbons from the original glucose into carbon dioxide so that we can obtain all the energy possible from them. The problem here is that to completely oxidize the carbons from acetyl-CoA generates some difficult chemistry problems.
The solution is to combine acetyl-CoA with another compound to make oxidation to CO2 feasible with the standard chemistries that we already know. In the citric acid cycle, the same five chemistries are used, but several enzymes combine steps - this makes them look more complicated than they are. We will look ar each one and break them down into smaller reactions so that we can see what is going on in each case more clearly.
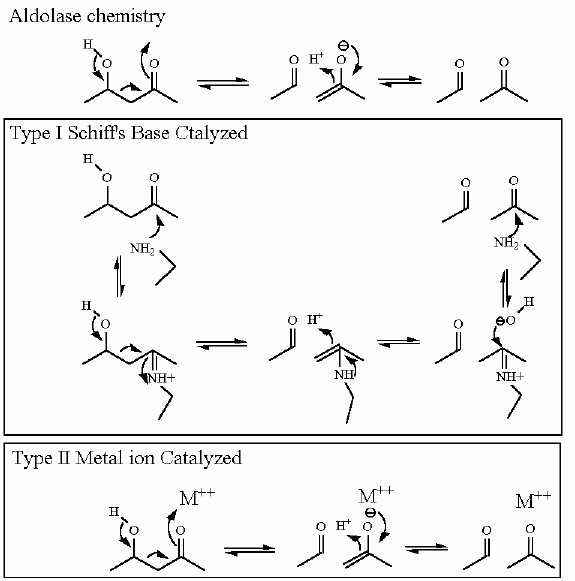
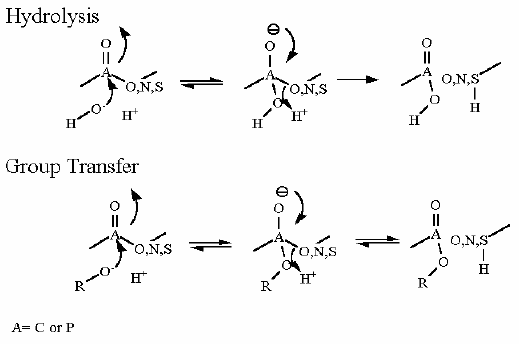
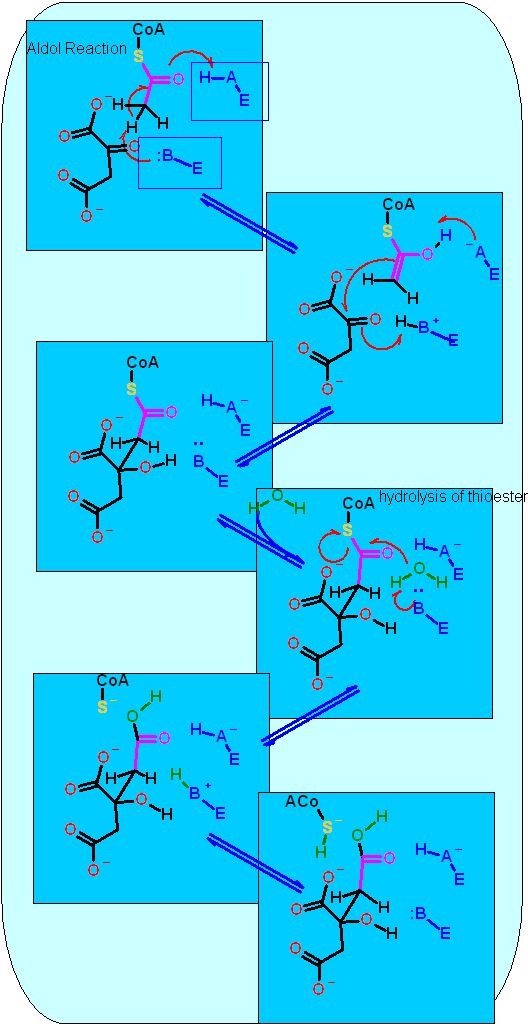
The citric acid cycle starts with oxaloacetate and ends with oxaloacetate (the same compound). As you might guess this generates some issues with making sure that the cycle operates in the "correct" direction. There are several reactions that enforce this direction. Citrate Synthase is one of them. While the aldol chemistry is readily reversible and has a ΔG°' near zero, the second reaction that occurs on the same enzyme active site is an hydrolysis which we know full well by know can really only proceed in one direction in water.
The aldol chemistry here complies with the same rules that all aldol chemistry must - The slight twist is that the thioester of acetyl-CoA acts as one of the "ketones" that is necessary for this reaction. Look for this in the reaction mechanism below and compare to the "standard" aldol chemistry.
|
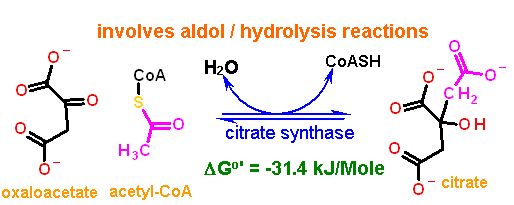

 This carbon is at the same oxidation state as an organic acid.
This carbon is at the same oxidation state as an organic acid.