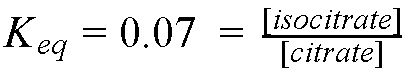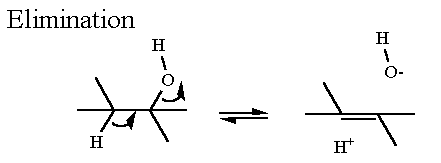|
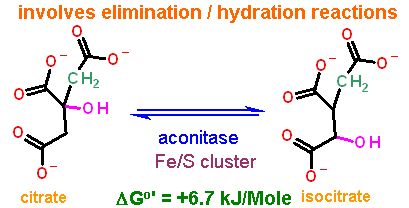
Isocitrate is an isomer of citrate. They have the same chemical formula, but different structures. However, the reaction type to generate isocitrate is NOT an isomerization - recall this has a specific meaning in the biochemical reactions. RATHER, this is a combination of two "elimination" reactions: one "forward" to remove the water and make a C=C bond and the other a "reverse" reaction to put the water back on across the double bond.
Elimination reactions usually require a metal. This is true here as well, but instead of a single metal ion like Mn2+ that was used in glycolysis, aconitase uses a complex metal ion - an Fe4S4 cluster. Clusters like these are frequently used in redox or electron transfer reactions and we will see numerous examples of this in the oxidative phosphorylation section of this module. In aconitase there is no redox - it is just being the strong electron puller necessary to aid an elimination reaction
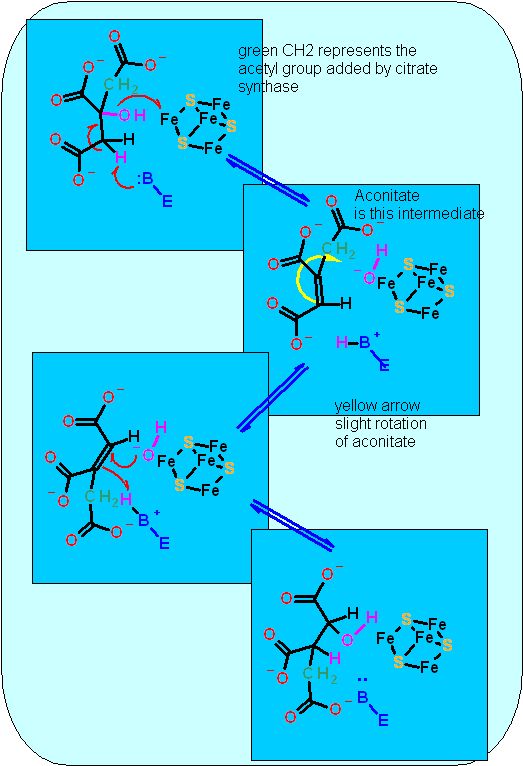
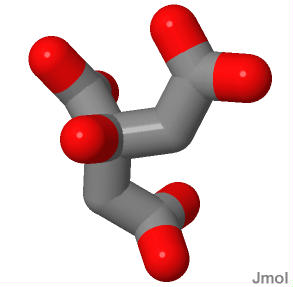 Shown here is a 3D representation of citrate the hydroxyl group is pointing out of the monitor toward you.It is not a chiral compound. There are only 3 unique substituents: the "arms" going up to the right and down look the same (they are both acetyl groups). You cannot tell by looking at this picture which of these were the acetyl group just put on by citrate synthase. Yet Aconitase CAN. While citrate is not chiral, neither is it symmetrical. The -OH group always gets moved AWAY from the acetyl group that was just put on. In the overall reaction at the top of the page I have labeled the methyl group of the "new" acetyl group in green. Shown here is a 3D representation of citrate the hydroxyl group is pointing out of the monitor toward you.It is not a chiral compound. There are only 3 unique substituents: the "arms" going up to the right and down look the same (they are both acetyl groups). You cannot tell by looking at this picture which of these were the acetyl group just put on by citrate synthase. Yet Aconitase CAN. While citrate is not chiral, neither is it symmetrical. The -OH group always gets moved AWAY from the acetyl group that was just put on. In the overall reaction at the top of the page I have labeled the methyl group of the "new" acetyl group in green.
Since citrate is not chiral there was some, there was some discussion initially regarding how an enzyme could catalyze the "movement" of the -OH group always in the same direction. Regardless of which direction the -OH group is moved, the same compound is obtained. How is it then that an enzyme can always move the -OH in the same direction? The answer (a revelation at the time) is that citrate is held in the active site of the enzyme by at least three anchor points. Becuase of it tetrahedral shape and the lack of mobility of the anchors, it is held in exactly the same position every time... effectively the enzyme active site MAKES it chiral. This is not a surprise anymore... but before anyone knew how enzmyes worked this was a revelation.
|
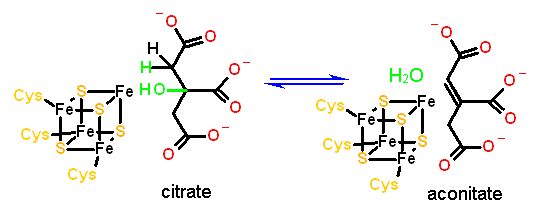 The Fe/s Center is required for these eliminations. Normally, all four irons are attached to an amino acid sidechain (usually cysteine). In this case one iron is exposed to the active site.
The Fe/s Center is required for these eliminations. Normally, all four irons are attached to an amino acid sidechain (usually cysteine). In this case one iron is exposed to the active site.

 Shown here is a 3D representation of citrate the hydroxyl group is pointing out of the monitor toward you.It is not a chiral compound. There are only 3 unique substituents: the "arms" going up to the right and down look the same (they are both acetyl groups). You cannot tell by looking at this picture which of these were the acetyl group just put on by citrate synthase. Yet Aconitase CAN. While citrate is not chiral, neither is it symmetrical. The -OH group
Shown here is a 3D representation of citrate the hydroxyl group is pointing out of the monitor toward you.It is not a chiral compound. There are only 3 unique substituents: the "arms" going up to the right and down look the same (they are both acetyl groups). You cannot tell by looking at this picture which of these were the acetyl group just put on by citrate synthase. Yet Aconitase CAN. While citrate is not chiral, neither is it symmetrical. The -OH group